Aethoxysklerol® – for treatment of all types of varicose veins, from spider veins to saphenous veins.
Studies confirm that 90% of the population shows evidence of varicose veins.1 Approximately 60% suffer from spider veins and reticular varicose veins and about 30% from larger varicose veins and their sequelae. The etiology of varicose veins includes many factors, which include age, inherent weakness of the connective tissue and female gender. In its early stages, this condition presents itself primarily as an esthetic problem, but even spider veins and reticular veins may affect personal well-being. Apart from the cosmetic concerns, the main symptoms are a feeling of tension in the legs, heavy and tired legs, pain and prickling, frequent night cramps, pruritus and edema.
If left untreated for a long period, varicose veins may result in more serious health issues such as phlebitis, skin irritation, hyperpigmentation, leg ulcers and deep vein thrombosis. However there is no need to wait until these occur.
“Sclerotherapy is considered to be the method of choice for the treatment of small-caliber varicose veins.”2
There is an effective, safe and gentle treatment for spider veins and varicose veins. Sclerotherapy, in which varicose veins are obliterated by injecting Aethoxysklerol® into the affected vein, is a well-established procedure which has been tried and tested throughout the world. The active pharmaceutical ingredient of the sclerosing agent Aethoxysklerol® is lauromacrogol 400 (polidocanol). In a recent FDA-approved, multi-center, randomized, double-blind clinical trial (EASI study),3 Aethoxysklerol® proved its efficacy as well as its excellent tolerance for the treatment of small varicose veins.
Aethoxysklerol® is suitable for all types of varicose veins and therefore can be adapted to the patient’s individual situation
The sclerosant Aethoxysklerol® is available in different concentrations ranging from 0.25% to 3% and can be administered both as a liquid and a sclerosing microfoam, depending on the size of the varicose veins or hemorrhoids to be treated.
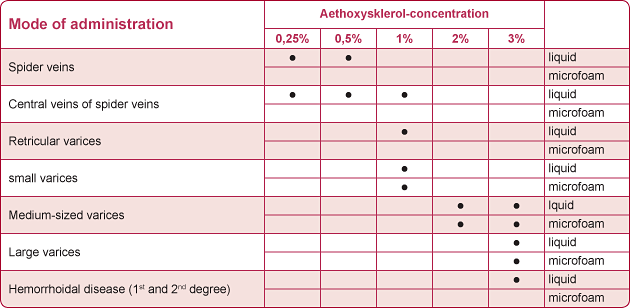
Mode of administration as indicated e.g. in the German summary of product characteristics
Please note: Not all concentrations and indications are available in every country. Trade names may vary from country to country. Information on this website is based on German Summary of Product Characteristics. Please refer to the labeling according to your national marketing authorization.
1 Rabe E. et al. Bonn vein study of the German Society of Phlebology: Phlebologie 2003; 32: 1-14.
2 Rabe E and Pannier F. Dermatol Surg. 2010; 36(Suppl 2): 968-75
3 Rabe E. et al. Sclerotherapy of telangiectases and reticular veins: a double-blind, randomized, comparative clinical trial of polidocanol, sodium tetradecyl sulfate and isotonic saline (EASI study). Phlebology 2010; 25: 124-131.
